Cellulose Acetate Phthalate is used as an enteric coating on capsules or tablets so they don't dissolve until they reach the small intestine. Enteric coatings are selectively insoluble substances – they won't dissolve in the acidic juices of the stomach, but they will dissolve in the higher pH (above pH 5.5) of the small intestine. Serrapeptase Rx capsules are enteric coated because the effectiveness of the drug will be reduced by stomach acids or enzymes if left unprotected.
Cellulose Acetate Phthalate
1. Nonproprietary Names
- BP: Cellacefate
- JP: Cellulose acetate phthalate
- PhEur: Cellulosi acetas phthalas
- USPNF: Cellacefate
2. Synonyms
Acetyl phthalyl cellulose; Aquacoat cPD; CAP; cellacephate; cellulose acetate benzene-1,2-dicarboxylate; cellulose acetate hydrogen 1,2-benzenedicarboxylate; cellulose acetate hydrogen phthalate; cellulose acetate monophthalate; cellulose acetophthalate; cellulose acetylphthalate.
3. Chemical Name and CAS Registry Number
Cellulose, acetate, 1,2-benzenedicarboxylate [9004-38-0]
4. Empirical Formula and Molecular Weight
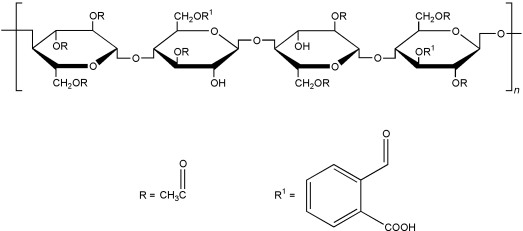
Cellulose acetate phthalate is a cellulose in which about half the hydroxyl groups are acetylated, and about a quarter are esterified with one of two acid groups being phthalic acid, where the remaining acid group is free. See Section 5.
5. Structural Formula
The PhEur 2002 (Suppl. 4.3) and USPNF 21 describe cellulose acetate phthalate as a reaction product of phthalic anhydride and a partial acetate ester of cellulose containing 21.5–26.0% of acetyl (C 2 H 3 O) groups, and 30.0–36.0% of phthalyl( o -carboxybenzoyl, C 8 H 5 O 3 ) groups.
6. Functional Category
Coating agent.
7. Applications in Pharmaceutical Formulation or Technology
Cellulose acetate phthalate (CAP) is used as an enteric film coating material, or as a matrix binder for tablets and capsules. 1 – 8 Such coatings resist prolonged contact with the strongly acidic gastric fluid, but dissolve in the mildly acidic or neutral intestinal environment.
Cellulose acetate phthalate is commonly applied to solid-dosage forms either by coating from organic or aqueous solvent systems or by direct compression. Concentrations generally used are 0.5–9.0% of the core weight. The addition of plasticizers improves the water resistance of this coating material, and formulations using such plasticizers are more effective than when cellulose acetate phthalate is used alone.
Cellulose acetate phthalate is compatible with many plasticizers, including acetylated monoglyceride; butyl phthalybutyl glycolate; dibutyl tartrate; diethyl phthalate; dimethyl phthalate; ethyl phthalylethyl glycolate; glycerin; propylene glycol; triacetin; triacetin citrate; and tripropionin. It is also used in combination with other coating agents such as ethyl cellulose, in drug controlled-release preparations.
Therapeutically, cellulose acetate phthalate has recently been reported to exhibit experimental microbicidal activity against sexually transmitted disease pathogens, such as the HIV-1 retrovirus. 9, 10
8. Description
Cellulose acetate phthalate is a hygroscopic, white to off-white, free-flowing powder, granule, or flake. It is tasteless and odorless, or might have a slight odor of acetic acid.
9. Pharmacopeial Specifications
See Table I.
10. Typical Properties
Density (bulk): 0.260 g/cm 3
Density (tapped): 0.266 g/cm 3
Melting point: 192°C. Glass transition temperature is 160–170°C. 11
Moisture content: 2.2%. Cellulose acetate phthalate is hygroscopic and precautions are necessary to avoid excessive absorption of moisture. 12 See also Figure 1.
Solubility: practically insoluble in water, alcohols, and chlorinated and non-chlorinated hydrocarbons. Soluble in a number of ketones, esters, ether alcohols, cyclic ethers and in certain solvent mixtures. It can be soluble in certain buffered aqueous solutions as low as pH 6.0. Cellulose acetate phthalate has a solubility of =10% w/w in a wide range of solvents and solvent mixtures; Table II and Table III.
Viscosity (dynamic): a 15% w/w solution in acetone with a moisture content of 0.4% has a viscosity of 50–90 mPa s. This is a good coating solution with a honey-like consistency, but the viscosity is influenced by the purity of the solvent.
11. Stability and Storage Conditions
Slow hydrolysis of cellulose acetate phthalate will occur under prolonged adverse conditions such as high temperatures and high humidity, with a resultant increase in free acid content, viscosity, and odor of acetic acid. However, cellulose acetate phthalate is stable if stored in a well-closed container in a cool, dry place.
12. Incompatibilities
Cellulose acetate phthalate is incompatible with ferrous sulfate, ferric chloride, silver nitrate, sodium citrate, aluminum sulfate, calcium chloride, mercuric chloride, barium nitrate, basic lead acetate, and strong oxidizing agents such as strong alkalis and acids.
13. Method of Manufacture
Cellulose acetate phthalate is produced by reacting the partial acetate ester of cellulose with phthalic anhydride in the presence of a tertiary organic base such as pyridine, or a strong acid such as sulfuric acid.
14. Safety
Cellulose acetate phthalate is widely used in oral pharmaceutical products and is generally regarded as a nontoxic material, free of adverse effects.
Results of long-term feeding in rats and dogs have indicated a low oral toxicity. Rats survived daily feedings of up to 30% in the diet for up to 1 year without showing a depression in growth. Dogs fed 16 g daily in the diet for 1 year remained normal.
15. Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Cellulose acetate phthalate may be irritant to the eyes, mucous membranes, and upper respiratory tract. Eye protection and gloves are recommended. Cellulose acetate phthalate should be handled in a well-ventilated environment; use of a respirator is recommended when handling large quantities.
16. Regulatory Status
Included in the FDA Inactive Ingredients Guide (oral tablets). Included in non-parenteral medicines licensed in the UK .
17. Related Substances
Cellulose acetate; hypromellose phthalate; polyvinyl acetate phthalate.
18. Comments
Any plasticizers that are used with cellulose acetate phthalate to improve performance should be chosen on the basis of experimental evidence. The same plasticizer used in a different tablet base coating may not yield a satisfactory product.
In using mixed solvents, it is important to dissolve the cellulose acetate phthalate in the solvent with the greater dissolving power, and then to add the second solvent. Cellulose acetate phthalate should always be added to the solvent, not the reverse.
Cellulose acetate phthalate films are permeable to certain ionic substances, such as potassium iodide and ammonium chloride. In such cases, an appropriate sealer sub-coat should be used.
A reconstituted colloidal dispersion of latex particles rather than solvent solution coating material of cellulose acetate phthalate is also available. This white, water-insoluble powder is composed of solid or semisolid sub-micrometer-sized polymer spheres with an average particle size of 0.2 µm. A typical coating system made from this latex powder is a 10–30% solid-content aqueous dispersion with a viscosity in the 50–100 mPa s range.
19. Specific References
-
Spitael J , Kinget R , Naessens K . Dissolution rate of cellulose acetate phthalate and Brönsted catalysis law. Pharm Ind 1980; 42 : 846–849.
-
Takenaka H , Kawashima Y , Lin SY . Preparation of enteric-coated microcapsules for tableting by spray-drying technique and in vitro simulation of drug release from the tablet in GI tract. J Pharm Sci 1980; 69 : 1388–1392. ( PubMed )
-
Takenaka H , Kawashima Y , Lin SY . Polymorphism of spray-dried microencapsulated sulfamethoxazole with cellulose acetate phthalate and colloidal silica, montmorillonite, or talc. J Pharm Sci 1981; 70 : 1256–1260. ( PubMed )
-
Stricker H , Kulke H . Rate of disintegration and passage of enteric-coated tablets in gastrointestinal tract [in German]. Pharm Ind 1981; 43 : 1018–1021.
-
Maharaj I , Nairn JG , Campbell JB . Simple rapid method for the preparation of enteric-coated microspheres. J Pharm Sci 1984; 73 : 39–42. ( PubMed )
-
Beyger JW , Nairn JG . Some factors affecting the microencapsulation of pharmaceuticals with cellulose acetate phthalate. J Pharm Sci 1986; 75 : 573–578. ( PubMed )
-
Lin SY , Kawashima Y . Drug release from tablets containing cellulose acetate phthalate as an additive or enteric-coating material. Pharm Res 1987; 4 : 70–74. ( PubMed )
-
Thoma K , Heckenmüller H . Effect of film formers and plasticizers on stability of resistance and disintegration behaviour. Part 4: pharmaceutical-technological and analytical studies of gastric juice resistant commercial preparations [in German]. Pharmazie 1987; 42 : 837–841. ( PubMed )
-
Neurath AR , Strick N , Li YY , Debnath AK . Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect Dis 2001; 11 : 17. ( PubMed )
-
Neurath AR , Strick N , Jiang S , et al . Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of ‘dead-end' gp41 six-helix bundles. BMC Infect Dis 2002; 21 : 6. ( PubMed )
-
Sakellariou P , Rowe RC , White EFT . The thermomechanical properties and glass transition temperatures of some cellulose derivatives used in film coating. Int J Pharm 1985; 27 : 267–277.
-
Callahan JC , Cleary GW , Elefant M , et al . Equilibrium moisture content of pharmaceutical excipients. Drug Dev Ind Pharm 1982; 8 : 355–369.
20. General References
-
Doelker E . Cellulose derivatives. Adv Polym Sci 1993; 107 : 199–265.
-
FMC Biopolymer . Technical literature: Aquacoat cPD, cellulose acetate phthalate aqueous dispersion, 1996.
-
Obara S , Mcginty JW . Influence of processing variables on the properties of free films prepared from aqueous polymeric dispersions by a spray technique. Int J Pharm 1995; 126 : 1–10.
-
O'Connor RE , Berryman WH . Evaluation of enteric film permeability: tablet swelling method and capillary rise method. Drug Dev Ind Pharm 1992; 18 : 2123–2133.
-
Raffin F , Duru C , Jacob M , et al . Physico-chemical characterization of the ionic permeability of an enteric coating polymer. Int J Pharm 1995; 120 2 : 205–214.
-
Wyatt DM . Cellulose esters as direct compression matrices. Manuf Chem 1991; 62 12 : 20, 21, 23.
21. Author
RW Fengl.
22. Date of Revision
October 22, 2002.